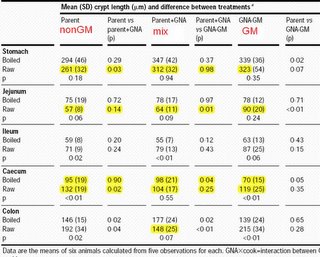
DATA TABLE FROM THE LANCET • Vol 354 • October 16, 1999
GMO Pundit explanation of data: Rats were fed three different diets, plain potato, potato plus GNA, and GM potato making its own GNA. GNA is a plant protein from snowdrops that is expected to interact with the gut. Data showing differences in gut thickness are coloured yellow. There are many differences, not just due to GM pototoes containing GNA lectin (right col.) but also non GM GNA mixed with potato (middle col.). In fact changes in gut thickness are rather randomly distributed over all the different treatments
Comment by Harry A Kuiper, Hub P J M Noteborn, and A C M Peijnenburg, Wageningen University and Research Centre,Netherlands.
Stanley Ewen and Arpad Pusztai report that, when fed to rats, GM potatoes containing the GNA lectin have proliferative and antiproliferative effects on the gut. They suggest that several of these effects are due to alterationsin the composition of the transgenic potatoes, rather than to the newly expressed gene product. However data on the composition of the different diets are not reported in the letter. Pusztai has released some of these details on the internet (http://www.rri.sari.ac.uk/gmo/ajp.htm ). These details indicate that the content of starch, glucose polymers, lectin[GNA], and trypsin and chymotrypsininhibitors in GM potatoes differed from that of the parental line.
Unfortunately, these differences have notbeen examined further by analysis of anextended range of lines, for evidence on whether these differences are attributable to the genetic modification or to natural variations.
Another shortcoming of the study is that the diets were protein deficient; they contained only 6% protein by weight. There is convincing evidence that short-term protein stress and starvation impair the growth rate, development, hepatic metabolism, and immune function of rats. Ewen and Pusztai say that the significant differences between diet groups invariables such as mucosalthickness or crypt length are evidence of the biological effects of the GM foods.
Such a claim is easy to make but difficult to prove, because no consistent patterns of changes were observed in the study.
Ingestion of potatoes may be associated with several adaptive changes in the gut because of the low digestibility of raw or partly refined potato starch. In rats caecal hypertrophy is a common response to short-term feeding of various poorly digestible carbohydrates, such as raw potatostarch. A physiological response of this nature is probably of little toxicological significance. Dose -response studies would
have helped in the assessment of consistency of response.
Effect of diets containing genetically modified potatoes expressing Galanthus nivalis lectin on rat small intestine
Stanley W B Ewen, Arpad Pusztai, p1353
See Commentaries pages 1314, 1315 of same Lancet issue
Editorial comment in this issue of the Lancet:
"Pusztai’s work has never been submitted for peer review,much less published, and so the usual evaluation of confusing claim and counter-claim effectivelycannot be made". This problem was underlined by our reviewers, one of whom, while arguing that the data were "flawed", also noted that, "I would like to see [this work] published in the public domain so that fellow scientists can judge for themselves . . . if the paper is not published, it will be claimed there is a conspiracy to suppress information". Publication of Ewen and Pusztai’s findings is not, as some newspapers have reported, a "vindication" ofPusztai’s earlier claims. On the contrary, publication of a paper after substantial review and revision provides a report that deserves further scientific attention. Such wider appraisal begins in this week’s Lancet with the commentary byHarry Kuiper and colleagues.
Update October 2006
Extended citations and criticisms of the Pusztai study (with special thanks to Klaus Amann)
GM food debate. Lancet, 354, 9191, pp 1726-1726
Lachmann, P. (1999)
Health risks of genetically modified foods. Lancet, 354, 9172, pp 69-69
Lachmann, P. (1999)
Lancet. 1999 Oct 16;354(9187):1314-5.
Genetically modified foods: "absurd" concern or welcome dialogue?
Horton R., The Lancet, London, UK.
Health risks of genetically modified foods
Vol 353 May 29, 1999 p 1811
THE LANCET Volume 353, Number 9167
http://gmopundit2.blogspot.com/2006/02/analysis-of-pusztai-study-on-gm.html
Mendel in the Kitchen: A Scientist's View of Genetically Modified Foods (2004)
Nina V. Fedoroff and Nancy Marie Brown Joseph Henry Press, Washington, D.C.
9. POISONED RATS OR POISONED WELLS
The increased public awareness of food allergy has arisen from a combination of three factors: reasoned concern, fear through ignorance, and political motivation.
—Bob Buchanan (2001)
On August 10, 1998, Arpad Pusztai was interviewed on the British TV show “World in Action.” Pusztai studied lectins, sugar-binding proteins found in peas and beans, cereals and potatoes. In his 35 years at the Rowett Research Institute in Aberdeen, Scotland, he had written three books on lectins and published 270 research papers. Because of his expertise, he had been asked to test the safety of a potato variety that had been genetically engineered to produce its own pesticide. That pesticide was a lectin from a flower, the snowdrop.
Pusztai fed the genetically modified potatoes to rats. His experiments, he told the television audience, showed that the GM potatoes damaged the rats’ immune systems and stunted their growth. He himself would not eat GM food, Pusztai said. He found it “very, very unfair to use our fellow citizens as guinea pigs.”...
Assessment of the food safety issues related to genetically modified foods
Harry A. Kuiper*, Gijs A. Kleter, Hub P. J. M. Noteborn and Esther J. Kok
The Plant Journal Volume 27 Page 503 - September 2001
Volume 27 Issue 6
Summary
International consensus has been reached on the principles regarding evaluation of the food safety of genetically modified plants. The concept of substantial equivalence has been developed as part of a safety evaluation framework, based on the idea that existing foods can serve as a basis for comparing the properties of genetically modified foods with the appropriate counterpart. Application of the concept is not a safety assessment per se, but helps to identify similarities and differences between the existing food and the new product, which are then subject to further toxicological investigation. Substantial equivalence is a starting point in the safety evaluation, rather than an endpoint of the assessment. Consensus on practical application of the principle should be further elaborated. Experiences with the safety testing of newly inserted proteins and of whole genetically modified foods are reviewed, and limitations of current test methodologies are discussed. The development and validation of new profiling methods such as DNA microarray technology, proteomics, and metabolomics for the identification and characterization of unintended effects, which may occur as a result of the genetic modification, is recommended. The assessment of the allergenicity of newly inserted proteins and of marker genes is discussed. An issue that will gain importance in the near future is that of post-marketing surveillance of the foods derived from genetically modified crops. It is concluded, among others that, that application of the principle of substantial equivalence has proven adequate, and that no alternative adequate safety assessment strategies are available.See also
The Full Monty on Animal Feeding Trials of GM Food and Similar Studies
Lancet. 2001 Jan 27;357(9252):309-10.
Press before paper - when media and science collide. C. N Stewart Jnr. Nature Biotechnology Vol 21 p353 (2003)
http://www.botanischergarten.ch/Pusztai/Pusztai-Myth-Royal-Comm-NZ.pdf
http://www.botanischergarten.ch/Pusztai/TraavikRoyalNZ.pdf
http://www.botanischergarten.ch/Pusztai/BibliographyWOS-Pusztai-20050716.pdf
No comments:
Post a Comment